Counting atoms with moles
April 1st, 2011 at 3:08 pm (Chemistry, History)
As you may have learned in high school chemistry, a mole is 6.022 x 1023 of something (usually something really tiny, like atoms). This seemingly arbitrary value is also known as Avogadro’s number. Why is it named after him, and how did that value come to be chosen?
Avogradro’s full name was Lorenzo Romano Amedeo Carlo Avogadro di Quaregna e di Cerret, and he had nothing to do with defining the value for a mole. Instead, he came up with the ideal gas law, which says that two samples of gas that occupy the same volume (with the same temperature and pressure) also contain the same number of molecules. It is most often encountered as pV = nRT. In 1909, 53 years after Avogadro died, Jean Perrin proposed naming the value of a mole after him.
Beyond Avogadro, why use 6.022 x 1023 as the basis for a mole? The history of this decision is an interesting illustration of what happens when a common value must be chosen for convenience, but there is no physical reason to prefer one over another (a similar situation arises for planetary coordinates; latitude has a well defined start point due to the axis of rotation, but longitude has to be given a start point arbitrarily). Originally, and quite logically, chemists used hydrogen (the simplest element, with only one proton) as a basis for computing atomic weights. They set H=1 and computed values for everything else relative to hydrogen. Later others proposed using oxygen as a standard (O=16), since oxygen is so prevalent and combines with so many things, and after decades of debate, in 1961 they finally settled on using carbon (C=12) as the standard. And so the value for a mole was born: the number of atoms in 12 grams of carbon-12 (isotopes matter!). (How do you count them though? See here.)
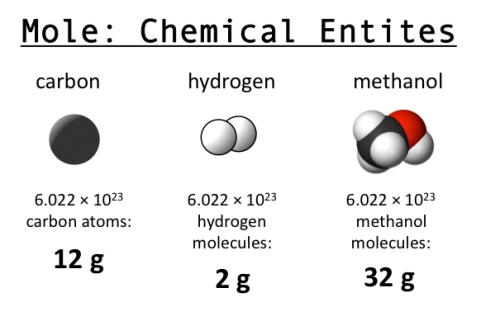 Why do we care? The mole is useful because of stoichiometry, one of my favorite words ever. If you know that you need 2 hydrogen atoms for every 1 oxygen atom to make water, then you know you need 2 moles of H for every 1 mole of O. A 2:1 ratio based on weight or volume won’t work, but you can convert moles into grams with knowledge about the atomic weight of each. This tells you exactly how much to mix to get the result you want, without wasting anything. Very efficient! (Image from Clear Science.)
Why do we care? The mole is useful because of stoichiometry, one of my favorite words ever. If you know that you need 2 hydrogen atoms for every 1 oxygen atom to make water, then you know you need 2 moles of H for every 1 mole of O. A 2:1 ratio based on weight or volume won’t work, but you can convert moles into grams with knowledge about the atomic weight of each. This tells you exactly how much to mix to get the result you want, without wasting anything. Very efficient! (Image from Clear Science.)
Even though it came from just 12 grams of carbon, a mole is a really, really big number. No, really. I love these analogies (from CDLI’s Chemistry 2202 course) that help us understand how big:
How big is a mole?
- one mole of peas is enough to cover Earth and 250 more planets the same size as Earth one metre deep in green peas
- a stack of one mole of pennies is tall enough to reach Proxima Centauri (the second closest star to Earth) and back again 7448 times
- a mole of marbles spread over the Earth would cover it to a depth of 80 km
- if you own a mole of dollars and you spend a billion dollars a day, then you could spend that amount per day for over a trillion years before you run out of money. (Earth has only been around for 4.5 billion years i.e. that’s 0.45% of a trillion years!)
For more molar fun, I highly recommend this mole lesson/activity. And six months from now, you can celebrate Mole Day: October 23, from 6:02 a.m. to 6:02 p.m.