Crystal gazing with the microscope
January 22nd, 2013 at 11:33 pm (Biology, Chemistry)
Adventure 2 in “Adventures with a Microscope” is titled “We Become Crystal Gazers.” The author continues:
We are not going to peer into such crystals as fortune tellers use, in which they claim to be able to predict that some rich relative is going to leave you money, or some equally nonsensical bosh.
No indeed, this is about chemistry. So I followed along, heated some water, and mixed up supersaturated solutions of various interesting substances from my kitchen. And wow, check it out! (Click to enlarge.)

Salt. Best viewed ~1 hour after deposition on the slide, with partial crystal formation; if you wait longer, the slide becomes crowded, and since the crystals are cubes, it’s hard to get them all in focus (too much relief!). I love this shot. It reminds me of Flatland.
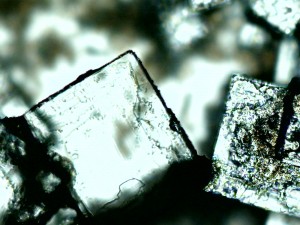
More salt, the next day, with larger crystals.
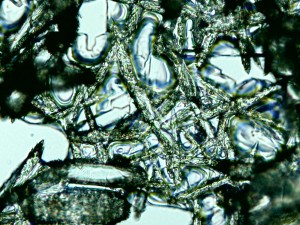
Baking soda, which apparently creates sparry crystals.

Sugar! This one took a full day to form interesting crystals. But wow, they are gorgeous!

More sugar, almost a butterfly-like configuration. I expect the symmetry is coincidence.
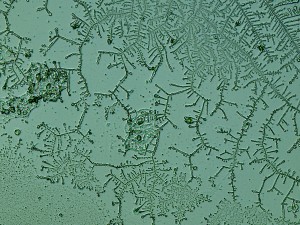
The biggest surprise for me — my saliva, 24 hours later, had created these awesome fractal patterns. At first I thought it might be nucleation following tiny scratches in the slide glass, but the fact that they’re fractal renders this unlikely. No clue what this is, but my best guess is that it’s just random diffusion patterns, like these manganese fractal patterns.
Aha, I found an article titled “Dendritic growth in viscous solutions containing organic molecules” which has these great examples:

Caption: “Crystal patterns of some body fluids: (a) saliva; (b) cerebrospinal fluid; (c) urine; (d) blood serum.”
My examples seem to match the “blood serum” image the best, but I assure you it was saliva. Not sure what’s going on in their sample (a)!

The more I panned across the expanse of fractal growth, the more it started looking like a map of a European city. Is it not marvelous?